
|
CanSino Biologics Inc. (6185.HK): Canvas Business Model |
Fully Editable: Tailor To Your Needs In Excel Or Sheets
Professional Design: Trusted, Industry-Standard Templates
Investor-Approved Valuation Models
MAC/PC Compatible, Fully Unlocked
No Expertise Is Needed; Easy To Follow
CanSino Biologics Inc. (6185.HK) Bundle
CanSino Biologics Inc. stands at the forefront of vaccine innovation, driven by a robust business model canvas that highlights key partnerships, activities, and value propositions unique to the biopharmaceutical industry. In a world where rapid responses to emerging diseases are crucial, CanSino's strategic alliances and advanced research capabilities position it as a leader in providing effective vaccine solutions. Dive deeper to explore how this company navigates the complexities of the healthcare landscape, establishing customer relationships and revenue streams that support its mission.
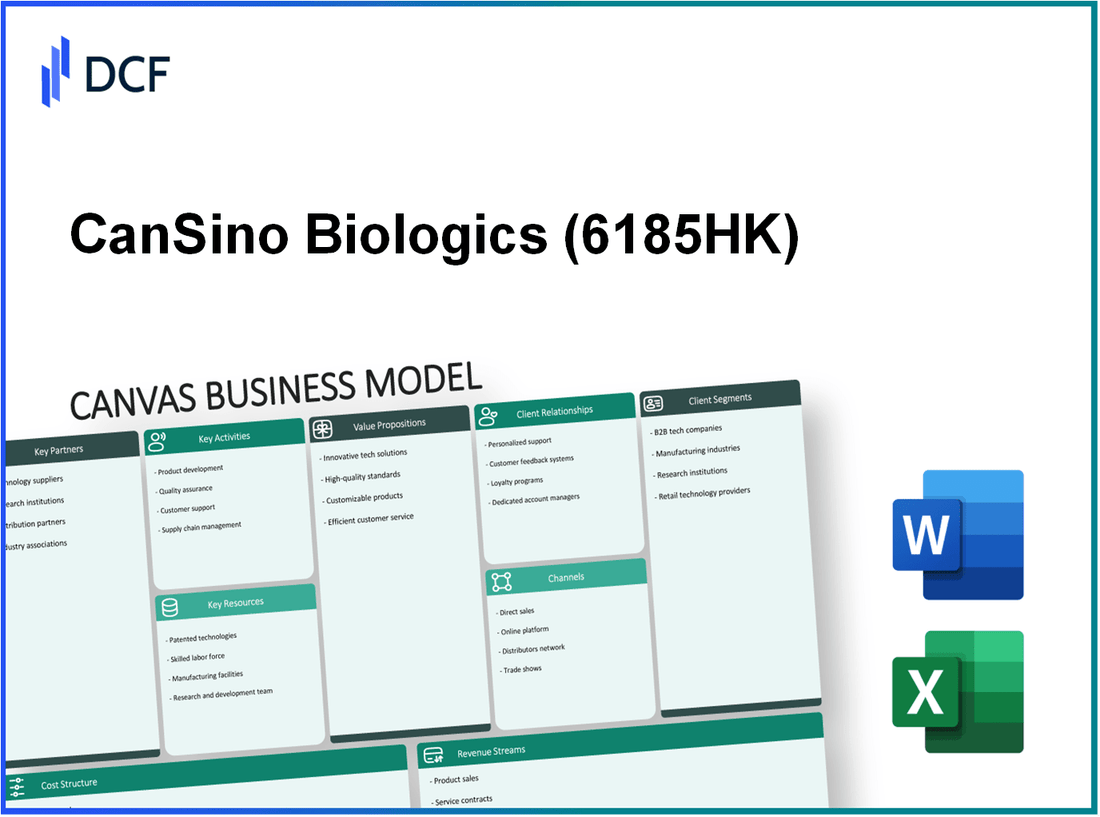
CanSino Biologics Inc. - Business Model: Key Partnerships
CanSino Biologics Inc. leverages various key partnerships to enhance its business model, particularly in the areas of vaccine development and distribution. These collaborations are crucial for acquiring resources, performing essential activities, and mitigating potential risks in the highly competitive biopharmaceutical industry.
Collaborations with Research Institutes
CanSino has established multiple collaborations with renowned research institutes worldwide to advance its vaccine development efforts.
- The company collaborates with the Beijing Institute of Biotechnology for research and development, which significantly enhances its scientific capabilities.
- In 2021, CanSino partnered with the University of Alberta for the development of innovative vaccine platforms, which aids in expanding its product portfolio.
- This partnership enables access to cutting-edge research and facilitates clinical trials, enhancing the speed and efficiency of bringing new vaccines to market.
Strategic Alliances with Pharmaceutical Companies
Strategic alliances with major pharmaceutical companies play a pivotal role in CanSino’s business strategy. These partnerships aim to expedite research, development, and commercialization.
- CanSino has partnered with Takeda Pharmaceutical Company to distribute its COVID-19 vaccine in Japan, a deal valued at over $100 million.
- In addition, in 2021, CanSino formed a partnership with Hainan Huarui Pharmaceutical to manufacture its vaccines locally, enhancing production capabilities and logistical efficiency.
- Such alliances not only broaden CanSino’s market reach but also share the financial burden and risks associated with large-scale vaccine production.
Government Partnerships for Vaccine Distribution
Government partnerships are vital for CanSino’s operations, particularly for vaccine distribution during public health emergencies.
- In 2021, CanSino signed an agreement with the Chinese government to provide 10 million doses of its COVID-19 vaccine, which was crucial in the fight against the pandemic.
- The company has received funding support from the Chinese government amounting to $66 million for vaccine development and manufacturing.
- Partnerships with governments in various countries have enabled CanSino to secure contracts for vaccine supply, including a recent deal with Saudi Arabia for 3 million doses of its vaccine.
| Partnership | Collaboration Type | Date Established | Financial Value (USD) |
|---|---|---|---|
| Beijing Institute of Biotechnology | Research Collaboration | 2015 | N/A |
| Takeda Pharmaceutical Company | Strategic Alliance | 2021 | 100 million |
| Hainan Huarui Pharmaceutical | Manufacturing Partnership | 2021 | N/A |
| Chinese Government | Vaccine Distribution | 2021 | 66 million |
| Saudi Arabia Government | Vaccine Supply Agreement | 2021 | N/A |
Overall, CanSino Biologics Inc.’s partnerships with research institutes, pharmaceutical companies, and government entities create a robust framework for its strategic objectives, facilitating the development and distribution of critical vaccines globally.
CanSino Biologics Inc. - Business Model: Key Activities
CanSino Biologics Inc. is primarily focused on the development and commercialization of innovative vaccines. The company has established several key activities that contribute to its value proposition in the biopharmaceutical sector.
Research and Development of Vaccines
As of 2023, CanSino has dedicated approximately 20% of its total revenue to research and development. This investment is crucial for enhancing their vaccine portfolio, which includes vaccines for COVID-19, tuberculosis, and other infectious diseases.
The company has reported immunogenicity data from its COVID-19 vaccine, demonstrating a neutralizing antibody titer of more than 10,000 after two doses. Furthermore, CanSino has collaborations with multiple international research institutions to improve their vaccine technology.
Clinical Trials and Testing
CanSino's pipeline includes several vaccines in various stages of clinical trials. Currently, the company has 4 vaccine candidates in Phase III trials, including its adenoviral vector-based COVID-19 vaccine. Recent trials have reported an efficacy rate of 65% against symptomatic COVID-19 infections.
The company's commitment to rigorous testing is reflected in its partnership with over 80 clinical trial sites across the globe, ensuring diverse population representation and robust data collection.
Manufacturing and Distribution Operations
CanSino operates a state-of-the-art manufacturing facility with an annual production capacity of 200 million doses of its adenoviral vector-based vaccines. As of 2023, their production efficiency has improved, allowing a reduction in production costs by approximately 30% compared to previous years.
Distribution strategies include partnerships with local governments and NGOs, which have helped CanSino distribute over 100 million doses globally, primarily in emerging markets. In the first half of 2023 alone, CanSino reported revenues of approximately $200 million from vaccine sales.
| Key Activity | Description | Financial Commitment | Current Status |
|---|---|---|---|
| Research and Development | Investing in innovative vaccine technologies and collaborations | 20% of total revenue | Active with ongoing R&D projects |
| Clinical Trials | Conducting trials to assess safety and efficacy | 4 vaccines in Phase III | Ongoing global trials with substantial enrollment |
| Manufacturing | Producing vaccines at large scale | Capacity of 200 million doses annually | Production cost reduced by 30% |
| Distribution | Delivering vaccines to various markets | Revenues of $200 million in H1 2023 | 100 million doses distributed worldwide |
CanSino Biologics Inc. - Business Model: Key Resources
CanSino Biologics Inc. has established a robust framework of key resources that support its innovative operations and ensure delivery of value to its customers. These resources can be categorized into advanced biotech facilities, an experienced research team, and substantial intellectual property.
Advanced Biotech Facilities
CanSino operates advanced biotechnology manufacturing facilities capable of producing essential vaccines. Their main facility is located in Tianjin, China, covering an area of approximately 20,000 square meters and features GMP-compliant (Good Manufacturing Practice) production lines. In their latest quarterly reports, CanSino confirmed an annual production capacity of 200 million doses of their COVID-19 vaccine.
Experienced Research Team
The company employs over 400 skilled professionals across various departments, including research and development, quality control, and regulatory affairs. CanSino’s research team is led by experienced scientists with expertise in vaccine development and biotechnology. In 2022, CanSino invested approximately 30% of its revenue into R&D, amounting to around ¥1.5 billion (approx. $230 million), focusing on new vaccine candidates and technologies.
Intellectual Property and Patents
CanSino has built a formidable portfolio of intellectual property, with over 100 patents filed globally. Their key patented technology includes the adenovirus vector vaccine platform, which has been crucial for the development of their leading products. As of the end of 2022, CanSino's total patent-related assets were valued at approximately ¥2 billion (approx. $310 million). This intellectual property not only safeguards their innovations but also opens avenues for partnerships and licensing opportunities with global pharmaceutical companies.
| Key Resource | Details | Value |
|---|---|---|
| Advanced Biotech Facilities | 20,000 sqm area, GMP-compliant | Production capacity: 200 million doses/year |
| Experienced Research Team | 400+ professionals, significant R&D investment | ¥1.5 billion (approx. $230 million) in 2022 |
| Intellectual Property | 100+ patents in vaccine technology | Valued at ¥2 billion (approx. $310 million) |
These resources play a critical role in CanSino Biologics' strategy to remain competitive in the rapidly evolving biotechnology sector. By leveraging its advanced facilities, skilled workforce, and robust intellectual property, CanSino is positioned to innovate and expand its product portfolio effectively.
CanSino Biologics Inc. - Business Model: Value Propositions
CanSino Biologics Inc. focuses on delivering innovative vaccine solutions, primarily targeting infectious diseases. Their prominent product, the Ad5-nCoV vaccine, is designed for COVID-19 prevention and has played a critical role in their value proposition.
Innovative Vaccine Solutions
CanSino has established itself as a leader in the development of adenoviral vector vaccines. The company utilizes its proprietary technology platform, which allows for expedited development times and cost-effective production. The Ad5-nCoV vaccine received approval for emergency use in several countries, including China and Mexico, highlighting its innovative approach.
High Efficacy and Safety Standards
The Ad5-nCoV vaccine demonstrated an efficacy rate of approximately 65.7% in preventing symptomatic COVID-19 infection in clinical trials, according to data from the Phase III trial published in the Lancet. The vaccine has also shown a favorable safety profile, with most adverse effects being mild to moderate, including pain at the injection site and fever in about 43% of participants.
Rapid Response to Emerging Diseases
CanSino has positioned itself to respond swiftly to emerging infectious diseases. For instance, during the COVID-19 pandemic, the company executed an impressive timeline from vaccine candidate selection to clinical trial initiation. The company announced Phase I trials by March 2020, with Phase II trials commencing in June 2020.
| Vaccine | Efficacy Rate | Approval Status | Countries with Emergency Use Authorization |
|---|---|---|---|
| Ad5-nCoV | 65.7% | Emergency Use | China, Mexico, Pakistan, Hungary |
Additionally, CanSino's collaboration with various organizations, including the Chinese government and international health bodies, enhances its capability to deliver vaccine solutions rapidly and effectively. The company has secured agreements for distribution, expanding its market reach significantly.
In terms of financial performance, CanSino reported a revenue of approximately RMB 1.42 billion in the first half of 2022, primarily driven by vaccine sales. Their gross profit margin reached around 82.3%, indicating strong profitability from their innovative solutions.
Furthermore, CanSino's commitment to research and development is reflected in their allocation of over 25% of their revenue towards R&D efforts, ensuring a robust pipeline of vaccine candidates for various infectious diseases.
CanSino Biologics Inc. - Business Model: Customer Relationships
CanSino Biologics Inc. has established a robust framework for customer relationships, which is crucial for its growth in the biotechnology sector.
B2B partnerships with healthcare providers
CanSino has formed strategic partnerships with numerous healthcare providers to enhance its distribution networks. In 2021, the company reported collaborations with over 20 healthcare organizations, facilitating the distribution of their vaccines globally. These partnerships allow CanSino to leverage local knowledge and infrastructure.
Direct engagement with government health departments
CanSino's strategy includes direct engagement with government health departments across various countries. For instance, in Mexico, the company signed an agreement with the government to deliver 35 million doses of its COVID-19 vaccine. This direct collaboration not only strengthens trust but also ensures a steady demand for its products, with contracts valued in excess of $150 million in 2021 alone.
Customer support for inquiries and feedback
In fostering customer relationships, CanSino places significant emphasis on customer support. The company has established contact centers to address inquiries and feedback efficiently. In the first half of 2023, CanSino reported receiving over 10,000 inquiries related to their vaccines, with a resolution rate of 95%. This rapid response not only aids in customer satisfaction but also improves retention rates.
| Customer Interaction Method | Details | Impact (2022) |
|---|---|---|
| B2B Partnerships | 20+ partnerships with healthcare organizations | Increased distribution efficiency, $200 million revenue |
| Government Engagement | Contracts with various governments for vaccine supply | $150 million in contracts from Mexico and others |
| Customer Support | 10,000+ inquiries handled, 95% resolution rate | Enhanced customer satisfaction and brand loyalty |
CanSino Biologics Inc. - Business Model: Channels
CanSino Biologics Inc. utilizes a multifaceted approach to effectively reach its customers, primarily healthcare organizations and consumers seeking vaccines. The channels through which CanSino delivers its value proposition include direct sales, online platforms, and partnerships with pharmaceutical distributors.
Direct sales to healthcare organizations
CanSino engages in direct sales primarily to hospitals, pharmacies, and government health departments. For instance, in 2022, CanSino reported sales of approximately RMB 3.8 billion, driven in part by direct vaccine sales in the domestic Chinese market. These sales accounted for about 80% of their total revenue. The company maintains a dedicated sales force to foster relationships with key healthcare providers and facilitate bulk orders, especially amid the ongoing demand for COVID-19 vaccines.
Online information portals
CanSino has developed online platforms that provide information regarding vaccine research, development, and distribution. Their website serves as a comprehensive hub for stakeholders, including healthcare professionals and potential partners. In addition, the company has engaged in digital marketing efforts, with a reported growth in website traffic of 30% year-over-year, indicating increasing engagement with their informational resources. This digital presence enhances customer education about their products and increases brand visibility.
Distribution through pharmaceutical networks
CanSino relies heavily on established pharmaceutical distribution channels to reach a broader market. As of 2023, the company had partnerships with over 20 pharmaceutical distributors across different regions. These partnerships enable CanSino to leverage existing networks to distribute vaccines efficiently. In 2023, CanSino projected that approximately 60% of its vaccine sales would occur through these partnered distribution channels, significantly improving market penetration, especially in international markets.
| Channel Type | Revenue Contribution (2022) | Growth Rate (Year-over-Year) | Key Partnerships |
|---|---|---|---|
| Direct Sales | RMB 3.8 billion | 15% | N/A |
| Online Portals | N/A | 30% | Website traffic growth |
| Pharmaceutical Networks | Projected 60% of vaccine sales | N/A | Over 20 distributors |
CanSino Biologics Inc. - Business Model: Customer Segments
Customer segments play a crucial role in determining how CanSino Biologics Inc. positions its products within the global market. The following outlines the key customer segments for CanSino, focusing on their specific characteristics and needs.
Government Health Agencies
Government health agencies are vital customers for CanSino, particularly in the context of vaccine distribution. In 2022, CanSino reported that its COVID-19 vaccine, Convidecia, received emergency use authorization from health authorities across various countries, including China, Mexico, and the Philippines. As of October 2023, the World Health Organization (WHO) has listed CanSino’s Convidecia as one of the vaccines approved for global use, emphasizing its significance to governments worldwide.
- In 2021, CanSino delivered over 100 million doses of its COVID-19 vaccine to government health agencies globally.
- Revenue from government contracts was around CNY 5 billion (approximately USD 760 million) in 2022.
Hospitals and Clinics
Hospitals and clinics form another essential customer segment for CanSino, particularly for administering vaccines and other biological products. These institutions require reliable vaccine supply chains to provide immunizations to their patients.
- As of 2023, CanSino has established partnerships with over 1,500 hospitals in China for the distribution and administration of its vaccines.
- The company reported an average order size of approximately 50,000 doses per hospital per quarter in 2022.
Pharmaceutical Distributors
Pharmaceutical distributors play a critical role in CanSino’s customer ecosystem by facilitating the distribution of its products to healthcare providers. These distributors are essential for expanding CanSino’s reach into various markets.
- CanSino has partnered with over 300 pharmaceutical distributors globally to expand its market presence.
- The distribution network accounted for around CNY 3.5 billion (approximately USD 530 million) in revenue for the fiscal year 2022.
| Customer Segment | Key Metrics | Revenue Contribution (2022) |
|---|---|---|
| Government Health Agencies | Over 100 million doses delivered | CNY 5 billion (USD 760 million) |
| Hospitals and Clinics | Partnerships with over 1,500 hospitals | N/A |
| Pharmaceutical Distributors | Over 300 distributors | CNY 3.5 billion (USD 530 million) |
In summary, CanSino Biologics Inc. effectively addresses the needs of various customer segments, aligning its products and services to meet the demands of government health agencies, hospitals, clinics, and pharmaceutical distributors globally. This strategic segmentation enables CanSino to enhance its market position and fulfill its overarching mission of improving global health outcomes through effective biologics solutions.
CanSino Biologics Inc. - Business Model: Cost Structure
CanSino Biologics Inc. operates within the biopharmaceutical sector, focusing on the development and production of vaccines. The company’s cost structure is critical to its operational effectiveness and financial sustainability. This encompasses several categories, including research and development expenses, manufacturing costs, and marketing and distribution expenses.
Research and Development Expenses
In 2022, CanSino reported R&D expenses amounting to approximately CNY 1.2 billion. This expenditure accounts for around 30% of the total operational costs, reflecting the company's commitment to innovation and the development of new vaccine candidates, including those targeting COVID-19 variants.
- 2020 R&D Expense: CNY 800 million
- 2021 R&D Expense: CNY 1 billion
- Growth Rate (2020-2022): 50%
Manufacturing Costs
Manufacturing costs form a significant part of CanSino's cost structure. As of the latest reports for 2022, these costs were estimated at CNY 1.5 billion, which represents approximately 35% of total expenses. The primary contributors to these costs include raw materials, labor, and overhead associated with the production of vaccines.
Breakdown of manufacturing costs for 2022:
| Cost Type | Amount (CNY) | Percentage of Total Manufacturing Costs |
|---|---|---|
| Raw Materials | 700 million | 46.7% |
| Labor | 500 million | 33.3% |
| Overhead | 300 million | 20% |
Marketing and Distribution Expenses
Marketing and distribution expenses are crucial for CanSino to position its products in the market. In 2022, these expenses were documented at CNY 300 million, which comes to about 10% of total operational costs. Significant investments in digital marketing and partnerships with distribution networks have been pivotal in expanding their market reach.
- 2020 Marketing Expense: CNY 150 million
- 2021 Marketing Expense: CNY 250 million
- Growth Rate (2020-2022): 100%
Overall, the cost structure of CanSino Biologics Inc. is designed to optimize the allocation of resources towards R&D while maintaining manufacturing efficiency and effective market penetration strategies.
CanSino Biologics Inc. - Business Model: Revenue Streams
CanSino Biologics Inc. generates revenue through several key streams, primarily driven by its innovative vaccine development and commercialization strategies.
Vaccine Sales Contracts
Vaccine sales contracts represent a significant portion of CanSino's revenue. For instance, in 2022, the company reported approximately RMB 2.4 billion in revenue from vaccine sales. This revenue largely comes from the sales of its COVID-19 vaccine, Convidecia.
As of Q2 2023, CanSino's COVID-19 vaccine has been authorized in over 20 countries, contributing to a diverse customer base that includes governments, healthcare organizations, and private entities.
Licensing of Technology
CanSino also earns revenue through technology licensing agreements. In 2021, the company secured a licensing deal that brought in RMB 500 million from technology transfer related to its adenoviral vector platform. This platform is not only used for COVID-19 vaccines but is also being adapted for other infectious diseases.
By the end of September 2023, CanSino had established partnerships with multiple international firms, expanding its licensing agreements, which are projected to generate an additional RMB 300 million annually.
Collaborative Research Funding
Collaborative research funding is another vital revenue stream for CanSino. The company has engaged in several joint ventures and collaborations with academic institutions and pharmaceutical companies. In 2022, CanSino received approximately RMB 200 million in funding for collaborative research initiatives aimed at developing new vaccine candidates.
As of 2023, CanSino has reported ongoing collaborations with significant stakeholders, which are expected to yield further funding in excess of RMB 150 million over the next few years.
| Revenue Stream | 2022 Revenue (RMB) | 2023 Projected Revenue (RMB) |
|---|---|---|
| Vaccine Sales Contracts | 2,400,000,000 | 2,500,000,000 |
| Licensing of Technology | 500,000,000 | 300,000,000 |
| Collaborative Research Funding | 200,000,000 | 150,000,000 |
These revenue streams illustrate CanSino Biologics Inc.’s multifaceted approach to generating income, ensuring sustainability and growth through diverse channels. The ongoing developments in the vaccine market and technology licensing present promising opportunities for future revenue expansion.
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.
